This study evaluated the effects of Palmitoylethanolamide (P.E.A.) supplementation on inflammation and anxiety in mice on a high-fat diet (HFD).
Key Points:
The addition of P.E.A. to a HFD had a number of protective effects in mice:
- Anxiety-like behavior caused by a HFD was resolved
- Systemic inflammation was reduced
- Brain was protected from inflammatory damage
19 Week Study Evaluates P.E.A. Supplementation in Mice on a High-Fat Diet (HFD)
Male mice were randomly divided into three groups (n=11 per group)
- Control group (STD): Chow diet + vehicle
- HFD group: HFD + vehicle
- HFD + PEA group: HFD + P.E.A. (ultra-micronized P.E.A., 30 mg/kg/day)
The vehicle or P.E.A. was added to the diets 12 weeks after the mice had been divided into groups and fed the respective chow or high-fat diets. The mice continued to receive the vehicle or P.E.A. for 7 weeks.
The STD diet contained 17% fat without sucrose.The HFD contained 45% fat and 7% sucrose.
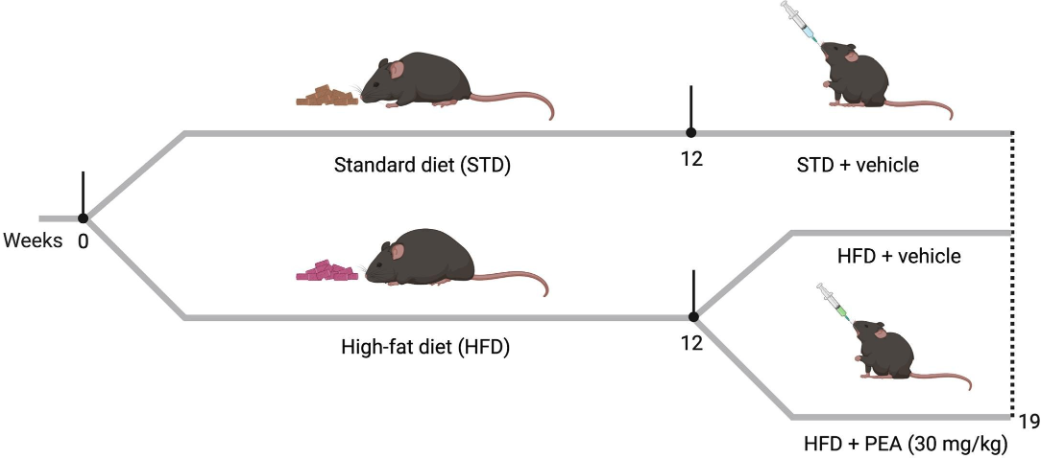
Experimental Design. After 12 weeks on diet, the STD group received the vehicle, while the HFD groups received either vehicle or ultra-micronized P.E.A. (30 mg/kg/day) in addition to their respective diets for seven weeks.
P.E.A Reduced Anxiety-Like Behavior Induced by HFD
The researchers used the open field test to measure anxiety-like behaviors in the mice.
Obese mice showed higher anxiety by staying at the edges of the test area and hesitating to explore the center zone. This was reversed with PEA treatment, where the mice explored more, covered greater distances and entered the center more often, similar to mice on a standard diet.
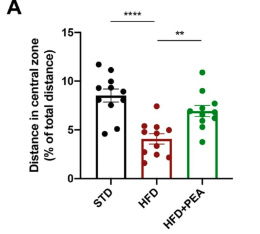
The red bar represents mice on a HFD; they traveled a shorter distance in the central zone compared to control mice on a standard diet (black bar). When supplemented with P.E.A., mice on a HFD showed increased distance traveled in the center zone (green bar).
“Here, we demonstrate the counteractive effect of PEA on anxiety-like behavior induced by HFD in mice.”
P.E.A. Protected Against Systemic Inflammation Induced by a HFD
The HFD significantly increased systemic inflammation.
“Here, we show that HFD animals exhibited altered serum parameters, characterized by elevated levels of cytokines (TNF-α, IL-1β), chemokines (MCP-1), and endotoxemia, defining metainflammation.”
However, supplementation with P.E.A. suppressed HFD-induced inflammation.
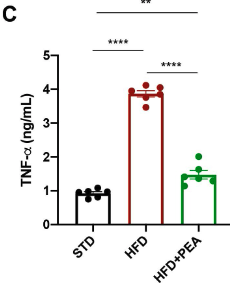
The mice on a HFD had significantly increased blood levels of the inflammatory cytokine, TNF-α (red bar) compared to mice on a standard diet (black bar). Supplmentation with P.E.A. blunted the HFD-induced increase in TNF-α (green bar).
P.E.A also suppressed the increase in the inflammatory markers LPS, MCP-1, and IL-1β observed in the un-treated mice on a HFD.
“In particular, we showed that PEA limited the endotoxemia induced by HFD, reducing the serum levels of LPS and the chemoattractant MCP-1. Furthermore, PEA confirmed its remarkable anti-inflammatory properties, as demonstrated by the reduction of serum TNF-α and IL-1β.”
P.E.A. Protected the Brain from Inflammatory Damage
P.E.A. improved brain health by reducing inflammation and strengthening the brain’s protective barrier.
Inflammatory proteins in the brain were increased in mice on a HFD. Treatment with P.E.A. significantly reduced all measured inflammatory markers.
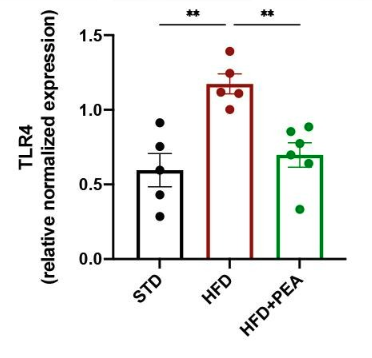
This figure represents the level of TLR4, an inflammatory protein, in the brains of the mice. Mice on a HFD (red bar) showed a dramatic rise in the levels of TLR4 in the brain compared to mice on a standard diet (black bar). When mice on a HFD were treated with P.E.A. (green bar), the levels of TLR4 did not rise a significant amount.
“Therefore, the reduced expression of TLR4 by PEA is consistent with the shutdown of the inflammatory cascade.”
P.E.A. also maintained the blood brain barrier (BBB) in mice on a HFD.
The BBB is a protective layer that controls what substances can enter the brain. It helps to protect the brain from harmful substances and maintain the delicate balance of chemicals in the brain.
“The detrimental process of neuroinflammation triggered by HFD caused the loss of BBB integrity, as shown by the reduced transcription of Tjp1, whose levels were improved by PEA.”
Conclusion
This study showed that P.E.A. protected mice from HFD-induced anxiety-like behavior and inflammation.
“As our data demonstrated, the blunted synthesis of pro-inflammatory cytokines by PEA can stop this vicious cycle among brain immune cells, limiting the progression of enduring neuroinflammation.”
