A pilot study tested single doses of intravenous (IV) NAD+ and NR, as well as oral NR, in healthy adults.
“This is the first study to clinically evaluate NR IV.”
Key Points
- NR infusions were faster and better tolerated than NAD+ infusions
- NR IV provided the fastest and most significant increase in NAD+ levels
- NAD+ IV did not significantly elevate NAD+ levels until 24 hours post-infusion
- NR displayed distinct metabolic patterns depending on administration route (IV vs. oral)
- NAD+ IV induced an inflammatory response, which was not observed in other groups
Single Doses of NAD+ IV, NR IV, and Oral NR Assessed in Healthy Adults
This study was a randomized, double-blinded, placebo-controlled pilot clinical trial, conducted in two parts.
Part One: Participants were randomly assigned to one of four groups, each receiving a single dose:
- NR IV (n=11; 500 mg; test group)
- NAD+ IV (n=10; 500 mg; active comparator group)
- Oral NR (n=10; 500 mg; bridge group)
- Saline IV (n=6; placebo control group)
Due to the distinct routes of administration (oral vs. IV), participants were not blinded to their treatment assignment in Part One.
Part Two: Participants were randomly assigned to one of two IV groups to ensure they remained unaware of which treatment they were receiving:
- NR IV (n=8; 500 mg)
- NAD+ IV (n=8; 500 mg)
Shorter Infusion Times and Improved Comfort with NR IV
While NAD+ infusions are common, there have been reports of them causing discomfort and requiring longer infusion times.
“Anecdotally, the [NAD+] infusions have been described as painful or uncomfortable, causing gastrointestinal disturbances, thereby necessitating an extremely slow rate of administration.”
In the first study group, the NAD+ IV infusion took the longest time at 3 hours, followed by NR IV at 2 hours, with saline IV having the shortest infusion time at 1.5 hours.
“In comparison to NAD+ IV, NR IV had a faster infusion time with superior tolerability.”
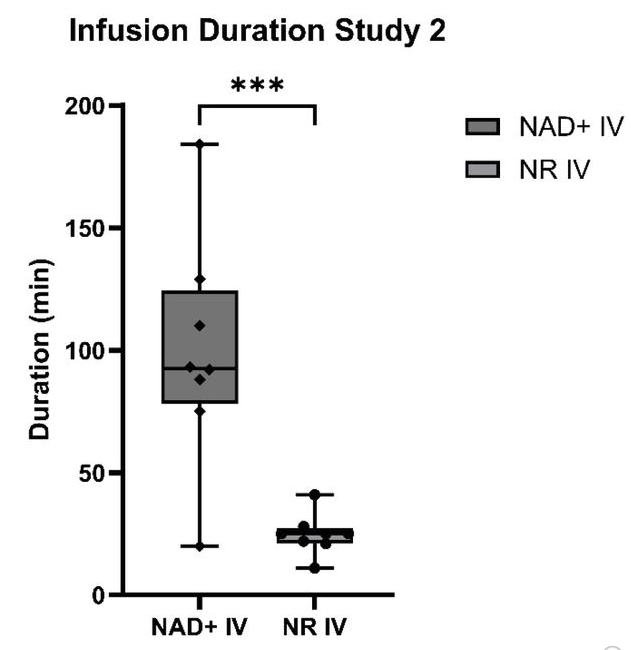
This graph illustrates the infusion rates for the second study group, which compared only NR and NAD+ IV infusions.
The NR IV infusion time was significantly shorter (right box, 24.75 minutes) compared to the NAD+ IV infusion time (left box, 98.88 minutes).
“The average infusion time for NR IV was ¼ of the time for NAD+ IV.”
Better Experience Reported with NR IV
The NR IV was generally well-tolerated, with the most common sensation being tingling in the mouth and extremities.
In contrast, NAD+ IV infusions were associated with a low comfort level.
Participants in the NAD+ IV group frequently reported anxiety, headaches, nausea, diarrhea, chest tightness, dizziness, and hot flashes.
“In both groups, symptoms were resolved once the infusion was completed.”
NR IV Showed Superior NAD+ Boost
Overall, NR IV was the most effective for raising NAD+ levels.
“NR IV appeared to promote the most robust increases in NAD+ concentration as measured by dried blood spot analyses.”
NR IV demonstrated a faster and more pronounced initial rise in NAD+ levels compared to NAD+ IV.
“NR IV increased NAD+ levels within 24 hours, which was surprisingly not observed with NAD+ IV. “
The line graph below depicts changes in whole blood NAD+ levels measured via dried blood spot analysis (DBS).
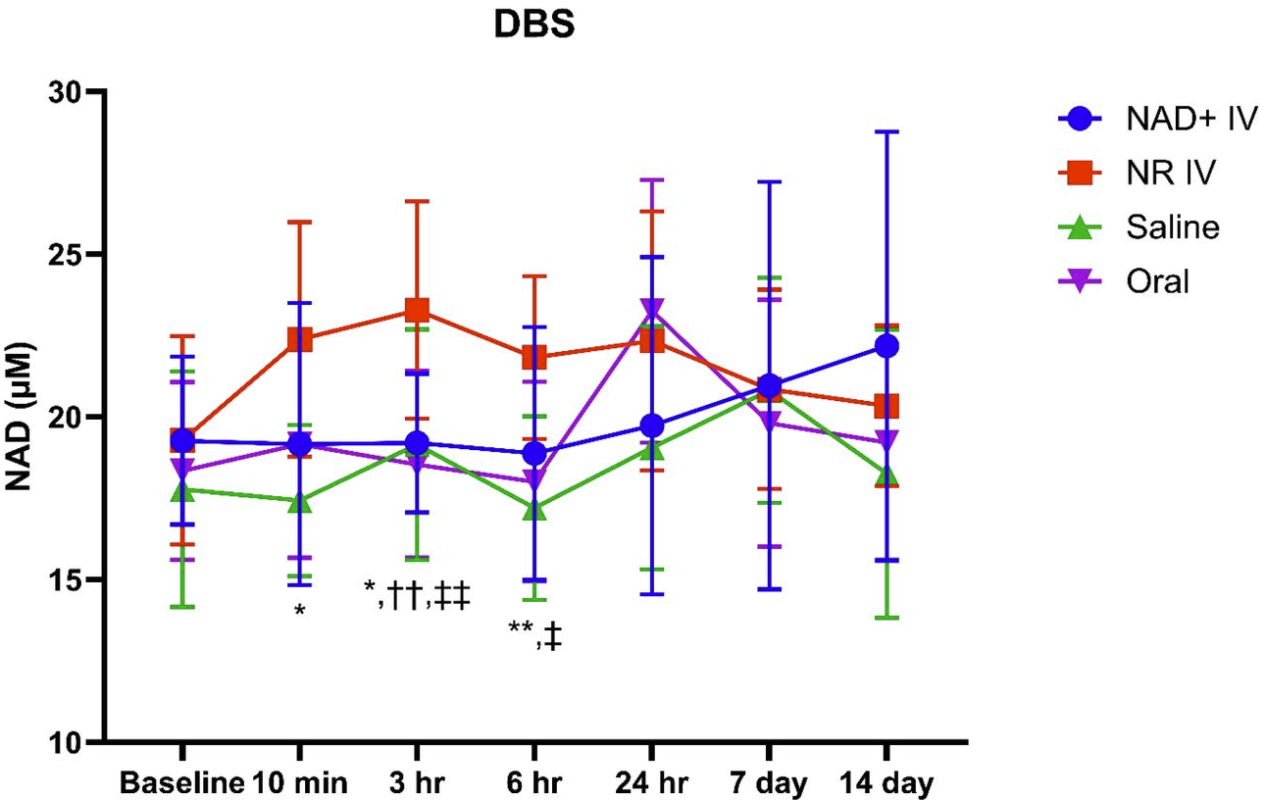
- NAD+ IV (blue line)
- No significant increase until 24 hours post-infusion
- Modest increases at 7 & 14 days, but not statistically significant
- NR IV (red line):
- Significant increase (20.7% from baseline) at 3 hours vs. placebo, NAD+ IV, oral NR.
- Near-significant increase at 6 hours vs. NAD+ IV; significant increase vs. placebo and oral NR
- While the initial rise from baseline outperformed other groups, total NAD+ levels were not significantly higher
- Oral NR (purple line):
- NAD+ levels peaked at 24 hours post-administration
The results highlight that the delivery method of these precursors affects their metabolism in the body.
Good Tolerance; Inflammation Noted with NAD+ IV
- Safety
- No significant changes were observed in key blood chemistry markers (e.g., electrolytes, liver function) for any intervention group compared to the control.
- No differences in blood pressure or heart rate were observed between the groups after treatment.
- No adverse events related to the treatments were observed
- Inflammation
- The NAD+ IV group showed an increase in inflammatory cells, suggesting the body may interpret the elevated NAD+ as a stressor, triggering an immune response
- This effect was not seen with NR IV or oral NR
Conclusion
This pilot study investigated NR IV administration for the first time, demonstrating its effectiveness.
“At 3 hours post-infusion, blood NAD+ levels were significantly higher in the NR IV group compared to the NAD+ IV group.”
NR IV offered a more favorable patient experience compared to NAD+ IV.
The discomfort associated with NAD+ IV was notably less with NR IV, which required significantly less time for infusion.
“In comparison to NAD+ IV, NR IV was infused faster, and the infusion experience was more tolerable.”
NR IV was well-tolerated and did not cause any differences in key safety measures.
“Overall, acute intravenous infusions of 500 mg NR were safe in the study participants with no attributable adverse events and only minor and transient infusion-related experiences.”
