
Yes, NMN can cross the cell membrane intact
This research was just published that found an enzyme named Slc12a8 that can transport a small quantity of Nicotinamide Mononucleotide (NMN) directly into some cells
However, this enzyme is only prevalent in the small intestine of older, sicker animals and humans.
The Slc12a8 enzyme was just discovered because it plays a very minor role, and does not transport a large enough quantity of NMN to have been noticed earlier.
More details about the research
Slc12a8 in the small intestine is important for transporting NMN from the gut into the circulation, affecting NAD+ levels in the small intestine and the systemic NMN supply in vivo.
Remarkably, the function of the Slc12a8 NMN transporter becomes crucial in aged individuals compared with young ones. In response to significant decreases in NAD+ levels, the aged ileum upregulates Slc12a8 expression and tries to maintain its NAD+ levels
When enough NMN is supplied, this feedback system can function adequately to maintain levels of NAD+ comparable to those in young
Research published Jan 7 2019 solves a mystery that has been the focus of intense speculation by scientists in this field for several years – how NMN enters the cell in order to become NAD+ and that it does not need to convert into NR (Nicotinamide Riboside) to do so.
Scientists at Washington University School of Medicine in St. Louis led by Shin-ichiro Imai, MD, PhD, have found the protein Slc12a8 rapidly transports NMN directly into cells.
Some key finding from this research:
- Slc12a8 transporter provides a fast, direct route to NAD+ inside cells
- Pathway is specific for NMN – not for NR, NAMN, or other metabolites
- Upregulated in older animals to compensate for decreased NAD+
- Stimulation of Slc12a8 transporter may increase NAD+ in cells
- Most prevalent in small intestine, liver, pancreas, and fat cells
- Effect on NMN supplements taken as capsules not quantified
Fast, direct transport of NMN inside cells
Before this study some researchers believed that NMN could not cross the cellular membrane intact. They claimed the only way for NMN to cross the cellular membrane was to first be converted to NR, cross the membrane, then convert back to NMN and on to NAD+.
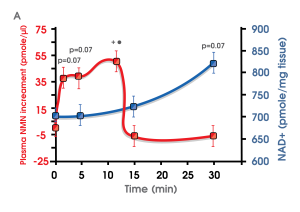 Dr Imai and his team long suspected there was a direct route for NMN to get into cells because NMN make the journey from the gut into the bloodstream and then into cells throughout the body within minutes much faster than has been observed in studies with NR, so Dr Imai was convinced there must be a direct route from external NMN and across cellular membranes.
Dr Imai and his team long suspected there was a direct route for NMN to get into cells because NMN make the journey from the gut into the bloodstream and then into cells throughout the body within minutes much faster than has been observed in studies with NR, so Dr Imai was convinced there must be a direct route from external NMN and across cellular membranes.
In this earlier study by Dr Imai and team published in 2016, mice were given a single dose of NMN in water.
NMN levels in blood showed it is quickly absorbed from the gut into blood circulation within 2’“3 min and then cleared from blood circulation and converted to NAD inside cells in the soleus muscle within 15 min
Slc12a8 is likely the reason NMN uptake is so quickly converted to NAD+.
The current study found mice that have the Slc12a8 gene “Knocked Down” (Slc12a8-KD), do not have the same speedy utilization.
“the fast uptake of NMN was completely abrogated in Slc12a8-knockdown (Slc12a8-KD) hepatocytes, whereas no significant reduction in NMN uptake was observed in Nrk1-knockdown (Nrk1-KD) hepatocytes (Fig. 1f), suggesting that Slc12a8 is necessary for the fast uptake of NMN in primary hepatocytes and that the observed increase in intracellular NMN is not due to the conversion of NR or nicotinamide into NMN.”
Also, mice missing the Slc12a8 gene were disadvantaged in NAD+ synthesis:
whole-body Slc12a8-KO mice display significant defects in direct, minute-order NMN transport and NAD+ biosynthesis
Pathway is specific for NMN – not for NR
Researchers used double labeled molecules of NMN, NR, and other NAD+ metabolites to trace the uptake into the cells.
Treating cells that overexpress the Slc12a8 gene with NMN results in uptake of approximately 4x more NMN inside the cells.
Treating the same cells with NR results in NO increase in NMN inside the cells.
In fact, even NAMN, which is nearly identical to NMN was not transported into the cells, confirming that this transporter is specific for carrying NMN across the cellular membrane.
“Here we show that the Slc12a8 gene encodes a specific NMN transporter. “
“We further show that Slc12a8 specifically transports NMN, but not nicotinamide riboside”
Stimulation of Slc12a8 transporter increases NAD+

The researchers were able to stimulate the Slc12a8 gene to “turn up” the quantity of Slc12a8 protein. This chart shows the increased NMN found in cells that “overexpress” the gene.
b, Uptake of 3H-NMN (25 μM, 37 °C) in control and Slc12a8-OE NIH3T3 cells (n = 12 biologically independent samples; analysed by ANOVA with Sidak’s test, *P = 0.0136, ***P = 0.0001).
With this in mind, Dr Imai’s lab already has identified small molecules that can stimulate production of the Slc12a8 NMN transporter, applied for patents, and licensed this technology to a company in Japan called Teijin Limited.
Upregulated in older animals to compensate for decreased NAD+
After mice were given oral gavage of 500 mg/kg NMN, intracellular NAD+ levels in small intestine of old mice were higher than that of young mice indicating that the upregulation of Slc12a8 plays an important role in counteracting age-associated NAD+ decline in the small intestine.
“expression of the Slc12a8 gene is upregulated in response to NAD+ decline, allowing cells to meet to an urgent demand for NAD+ biosynthesis”
Most prevalent in small intestine, liver, pancreas, and fat cells
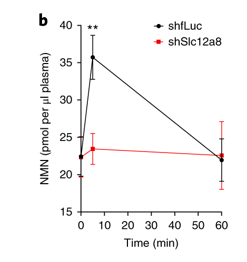
b, Plasma NMN levels after an oral gavage of NMN (500 mg per kg body weight) in control and Slc12a8-KD mice (n = 6 mice; B6 males at 3–4 months of age; analysed by ANOVA with Sidak’s test, **P = 0.0080).
Most of their test were with small intestine, as that is where Slca18 is most abundant, but is also found in liver, pancreas, and fat cells:
“Slc12a8 is highly expressed in the small intestine and pancreas and moderately expressed in the liver and white adipose tissue”
They also did some studies with liver cells that show a 90% reduction of NMN uptake with Slc12a8 Knockout:
When treated with 100 μM O18-D-NMN, Slc12a8-KO hepatocytes showed ~90% reduction in O18-D-NMN uptake compared with control wild-type hepatocytes at 5 min
In vivo validation of the NMN transporter
orally administering NMN (500 mg per kg body weight) to those mice, plasma NMN levels significantly increased at 5 min in the control mice, whereas they did not increase at all in the Slc12a8-KD mice
Instead, plasma nicotinamide levels tended to be higher
Slc12a8 maintains NAD+ levels in the aged gut
We found that the jejunum and ileum in 24-month-old mice also showed NAD+ decreases compared with 2-month-old mice. Consistent with this phenomenon, Slc12a8 expression was also significantly upregulated in the aged ileum
After NMN oral gavage, NAD+ levels were also increased in aged mice to levels close to those observed in young mice (Fig. 4e)
Slc12a8 KD to test importance in young vs old mice
Significant NAD+ decreases were detected in the ilea of the aged, but not young, Slc12a8-KD mice
slc12a8 increased in young mice, resulting in significant NAD+ increase
Shows slc12a8 increased importance to maintain NAD+ in aged animals
these results demonstrate that the upregulation of Slc12a8 contributes to the maintenance of NAD+ levels
Contradictions with earlier research?
This study (Liu,Rabinowitz) published in March 2018 showed that nearly all NMN and NR taken orally is degraded to NAM in the GI tract and liver, with none being delivered intact outside the liver.
This would seem to be contrary to the findings in the present study that show oral gavage effectivelydelivered NMN inside cells where it was quickly converted toNAD+
A few points that may explain this appearant discrepancy:
- The Liu study used fairly young mice.Slc12a8 transporter is not used/needed in young animals
- The Liu study did not publish data on NAD+ levels in small intestine after administering NMN
- Dr Imai points out this most recent study measured NAD+ metabolites in blood immediately, as they say freezing and thawing blood (which is what was done in the Liu study) destroys some NAD+ metabolites
Questions still remain
Discovery of this dedicated NMN transporter is intriguing, however
this study did not attempt to quantify the NMN that makes it to NAD+ intact thru the Slc12a8 transporter.
The authors do not in any way imply that ALL NMN supplements might be able to utilize this pathway.
Dr Sinclair, in this review of the Imai research says:
It is important to note that the discovery of an NMN transporter by no means diminishes the importance of uptake via dephosphorylation
We do not know:
- What percentage ofNMN supplements likely get degraded in the stomach before reaching small intestine.
- What percentage of NMN that makes it to the small intestine is able to utilize this transporter?
- How much of theNMN that is successfully converted toNAD+ from small intestine supply other tissues in the body?
- What is the range of NMN dosages that might be useful before Slc12a8 transporter is overwhelmed?
- What can be done to stimulate more Slc12a8 ?
Why we need to use Sublingual Delivery for NMN
Our knowledge of NAD+ biology continues to grow, and, no doubt, more surprises are in store down the road as the NAD+ story continues to evolve.
We now have proof there is a dedicated NMN transporter, itis upregulated in older animals to compensate for low NAD+ levels, and it is likely thereason NMN is found tobe metabolized to NAD+inside cells so much more quickly than NR.
But we do not know what percentage of any NMN supplements might be able to utilize this pathway to NAD+.
We also don’t know how effectively the body can transfer NAD+ from the small intestine to other tissues throughout the body.
NMN doesn’t require Slc12a8 to enter cells.It is an alternate pathway that is specific for NMN and most prevalent in small intestine, liver, and pancreas.
In some cells without Slc12a8, NMN needs to lose a phosphate (be converted to NR) to enter.However, that does not impede it from entering.
NMN uses a protein it finds readily available at the cell membrane to discard the phosphate.
It is not really an impassible barrier to entry that Chromadex likes to portray, but more like a checkpoint.Maybe like you are at a friends house, and need to remove your shoes before entering.
In a test tube, NR is faster to enter cells.But in vivo, it’s not.Taken in water or gavage, they raise NAD+ in the liver nearly the same amount at the same speed.
Taking NMN sublingual may put 30% or so in the blood in minutes, where it can enter cells throughout the body.If you swallow a capsule of NR , it takes and hour or more to go thru liver before some very small % can reach the bloodstream.
Now we know that with Slc12a8, some % of NMN can be converted to NAD+ in the small intestine, much quicker than NR from the liver.But we don’t know what % that is, and likely HUGE variation in it.
Conclusion
Prior to this new discovery, it was generally thought that NMN could not enter the cell directly and that it had to convert back into NR in order to do so.
We now know this is not true, and there most definately IS a dedicated transporter that is specific for NMN.
Without the Slc12a8 transporter,NMN capsules suffer the same fate as NR, and are almost completely digested to NAM in the stomach and Liver (Liu,Rabinowitz)
If the Slc12a8 protein is stimulated (as in older animals), some of that NMN can be transported directly into cells intact and increase NAD+.
However, there is no reason to believe that Slc12a8 can process all NMN that passes through the small intestine and liver – Sublingual delivery may be more effective capsules.
