The research presented below was in mice or rats.
NO HUMAN studies have been published on nasal administration of NAD+ or NAD precursors.
A recent review highlighted the therapeutic potential of NAD+ nasal spray for brain injuries.
“Intracellular NAD has a short half-life estimated to be 1 to 2 h; therefore, intranasal administration is preferable over systemic routes.”
Key Points
Animal studies investigating NAD+ delivered through nasal spray show benefits for brain injuries:
- Reduced brain injury in rats after stroke
- Prevented brain cell death in rats after head injury
- Slowed down the progression of brain infection in mice
Decreased Brain Injury After Stroke
A study found that intranasal NAD+ (10 mg/kg) increased brain NAD+ levels and reduced brain damage in rats after a stroke.
“The results showed that the NAD+ administration can significantly increase the NAD+ levels in the brains, suggesting that NAD+ can be delivered into the brains by this approach.“
Rats treated with NAD+ through the nose showed decreased formation of brain lesions (infarcts).
“Massive infarct formation was found in the brains of rats subject to ischemia-reperfusion, which was decreased by intranasal administration with 10 mg / kg NAD+”
Improved neurological function was also observed with nasal NAD+ treatment.
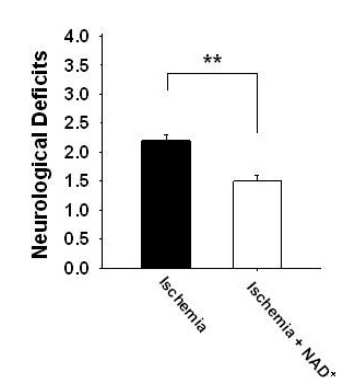
This figure illustrates the neurological function assessed 24 hours after the stroke. Rats receiving intranasal NAD+ treatment (white bar) exhibited markedly fewer neurological deficits compared to untreated rats (black bar).
Prevented Brain Cell Death After Head Injury
In another study, nasal NAD+ provided substantial protection in the hippocampus of rats following a traumatic brain injury:
- Restored NAD+ levels in the hippocampus
- Prevented the death of neurons
- Halted the activation of inflammatory cells
The figure below shows the degree of neuronal degeneration in specific areas of the hippocampus, including CA1, CA3, and the dentate gyrus (DG).
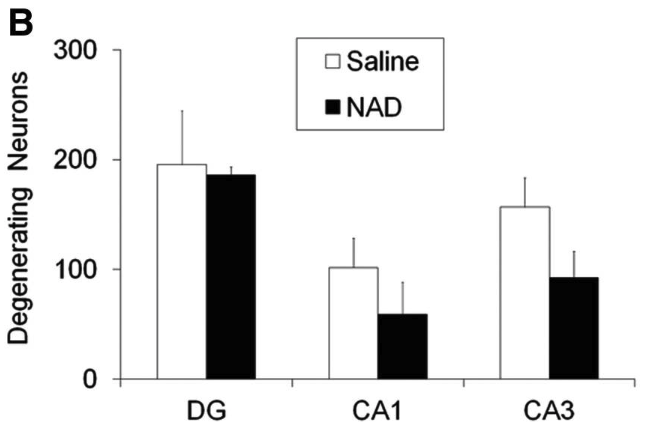
Rats treated with NAD+ through the nose (black bars) had fewer damaged neurons after a TBI compared to untreated rats (white bars) in the DG, CA1, and CA3 regions of the hippocampus.
Interestingly, injecting NAD+ didn’t protect neurons like the nasal spray, highlighting the importance of delivery methods.
Intraperitoneal injection of the same NAD+ concentration that we used for intranasal administration showed no neuroprotective effects on TBI-induced neuronal death.
Nasal sprays, unlike injections, avoid processing and breakdown by the liver, potentially delivering NAD+ to the brain more effectively.
Delayed the Progression of Brain Infection
A study in mice showed that intranasal delivery of NAD+, even when started after the onset of symptoms, can improve motor function and delay the progression of prion disease.
“Mice were treated daily with 30 mg/kg NAD+ by the intranasal route as a means to circumvent the blood–brain barrier.”
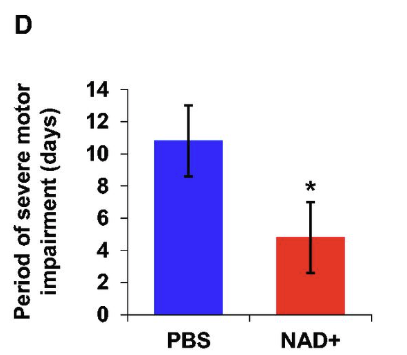
This figure shows the time period during which mice suffered severe motor impairment was significantly shorter in the NAD+ treated group (red bar) than in the control group (blue bar).
Conclusion
Intranasal delivery offers a promising approach to delivering neuroprotective compounds directly to the brain, bypassing the limitations of traditional routes.
“Compared to traditional routes, the nasal administration of drugs can direct the rapid CNS absorption to brain tissues, thereby circumventing the hepatic first-pass metabolism and gastric degradation and allowing fast onset of pharmacological action.”
This is supported by studies showcasing the effectiveness of intranasal NAD+ in aiding with brain injury recovery.
The research presented above was in mice or rats.
NO HUMAN studies have been published on nasal administration of NAD+ or NAD precursors.
