This study characterized mitochondrial stress response dysregulation in Alzheimer’s disease (AD) patients and evaluated the therapeutic potential of NMN in an AD mouse model.
Key Points
- The mitochondrial stress response is disrupted in AD patients
- NMN improved cognitive ability and protected brain cell integrity in AD mice
- Key pathways in the mitochondrial stress response (UPRmt and mitophagy) were enhanced by NMN
- The beneficial effects of NMN were dependent on the regulator protein, ATF4
Evaluation of the Mitochondrial Stress Response Pathway in AD Patients and as a Therapeutic Target for NMN in AD Mice
Plasma mitochondrial stress response markers were evaluated in AD (n=43) and healthy (n=46) participants.
The study used an AD mouse model to assess the impact of NMN treatment. Mice were randomized into the following groups and treated for two months:
- WT (VEH): Healthy mice with no NMN treatment
- WT (NMN): Healthy mice treated with NMN (i.p., 500 mg/kg/day)
- AD (VEH): AD mice with no NMN treatment
- AD (NMN): AD mice treated with NMN (i.p., 500 mg/kg/day)
After two months, the mice were evaluated for behavioral and molecular outcomes.
Plasma Markers of Mitochondrial Stress and Cell Cleanup are Disrupted in Alzheimer’s Patients
Researchers analyzed the plasma of individuals with Alzheimer’s disease (AD) and healthy controls. They found that specific stress response and cellular cleanup processes were linked to AD.
“Here, we report dyshomeostasis plasma UPRmt-mitophagy-mediated MSR profiles in AD patient samples.“
Specifically, AD patients demonstrated significantly higher levels of key mitochondrial stress response biomarkers, including ATF4, ATF5, CHOP, PINK1, and Parkin, compared to healthy controls.
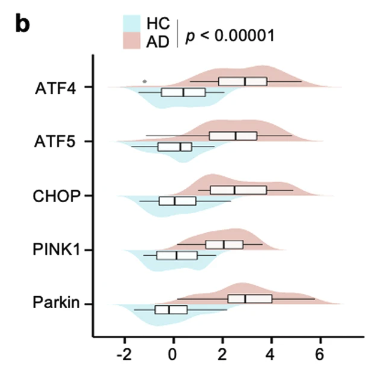
This figure shows that the levels of the mitochondrial stress response markers (ATF4, ATF5, CHOP, PINK1, and Parkin) were higher in AD patients (red) compared to healthy controls (blue).
Notably, elevated levels of ATF4 and PINK1 were linked to more severe memory and cognitive decline.
The study also revealed that the relationship between certain stress response proteins (ATF4, ATF5, CHOP) and an AD diagnosis was partially influenced by cellular cleanup proteins (PINK1 and Parkin).
Building on these findings, the researchers investigated NMN’s potential to modulate these pathways in an AD mouse model.
“While cumulative evidence suggests that increased mitochondrial stress response (MSR) may mitigate neurodegeneration in AD, explorations to develop a MSR-targeted therapeutic strategy against AD are scarce.”
NMN Improved Memory and Learning in AD Mice
Mice with AD treated with NMN showed improved learning, memory, and cognitive abilities in various behavioral tests.
The figure below shows the results from the Novel Object Recognition (NOR) test, which evaluates visual recognition memory and cognitive ability.
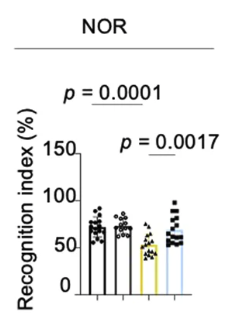
AD mice supplemented with NMN (blue bar) scored significantly higher than AD mice on a standard diet. Their scores were comparable to those of healthy mice on a standard diet (black bar) or supplemented with NMN (dark gray bar).
NMN supplementation also decreased amyloid-beta (Aβ) plaques and related proteins in the hippocampus, which are key markers of AD.
“These findings in transgenic mouse models of AD suggest a robust neuroprotective role of NMN against AD pathologies and cognitive deficits.”
The Mitochondrial Stress Response was Enhanced by NMN
Researchers studied two key ways cells maintain mitochondrial health in the mitochondrial stress response in the brains of mice with Alzheimer’s disease (AD):
- UPRmt: A response that helps repair and protect mitochondria when they are stressed
- Mitophagy: The process of removing damaged mitochondria
They found that NMN treatment increased the levels of several proteins involved in these pathways in the brains of AD mice, suggesting improved mitochondrial quality control:
- UPRmt chaperone proteins (protect and fold proteins): ATF4, ATF5, and CHOP
- UPRmt protease proteins (break down and remove damaged proteins): YME1L1, CLpP, and LONP1
- Mitophagy targets (help identify and remove damaged mitochondria): PINK1, Parkin, FUNDC1, OPTN, and Becline 1
NMN Protected Brain Cell Connections, Reduced Neuronal Damage and Brain Tissue Shrinkage
AD is characterized by the loss of connections between brain cells, known as synapse degeneration. The study found that NMN improved synaptic health in AD mice by:
- Increasing levels of synaptic proteins (synaptophysin and PSD-95), which are markers of healthy synapses
- Preserving the structure of dendritic spines, which are important for brain cell communication
NMN treatment also reduced brain cell damage and shrinkage in AD mice.
Additionally, NMN improved mitochondrial health and function by:
- Improving the balance of mitochondrial and nuclear proteins
- Decreasing mitochondrial damage and stress
- Boosting energy production (higher ATP levels)
“At the organismal level, NAD+ repletion with NMN supplementation ameliorates mitochondrial proteotoxicity, decreases hippocampal synaptic disruption, decreases neuronal loss, and brain atrophy in mice model of AD.”
NMN’s Benefits Are Mediated By the ATF4 Pathway
Researchers then wanted to see if NMN’s benefits were connected to the ATF4 pathway, which plays a role in cellular stress responses.
“ATF4 belongs to the activating transcription factor (TF) family and its activation is enhanced upon the stimulation of a diverse array of stresses, which could regulate the expression of multiple genes that are involved in oxidative stress, differentiation, metabolism, and development.”
When the ATF4 pathway was inhibited, NMN’s positive effects were lost:
- Memory, learning, and movement improvements were reversed
- The reduction in amyloid plaques (protein clusters associated with AD) was no longer evident
Conclusion
This study revealed that key processes involved in the mitochondrial stress response are impaired in the plasma of AD patients.
Treatment with NMN enhanced the mitochondrial stress response and improved cognitive function in an AD mouse model.
“We combined cell biology, molecular biology, and pharmacological approaches to unravel a novel molecular pathway by which NAD+-boosting agent nicotinamide mononucleotide (NMN) regulates MSR in AD models.”
Brain cell communication, neuronal health, and brain tissue integrity were preserved in AD mice treated with NMN.
The neuroprotective effects of NMN required the activation of the ATF4 pathway, a key regulator of the mitochondrial stress response.
“Our work provides evidence that mitochondria have an active role in the pathogenesis of AD, as reducing mitochondrial homeostasis via atf4 depletion in 5xFAD mice aggravates the hallmarks of the disease.”
“Conversely, boosting mitochondrial proteostasis by NMN decreases protein aggregation, restores memory performance, and delays disease progression, ultimately translating to increased healthspan.”
