The NAD World concept was introduced in 2009 to describe the complex connections between NAD+ metabolism and aging.
“NAD World is a systemic regulatory network that connects NAD+ metabolism, biological rhythm, and aging and longevity control in mammals.”
As research progresses, this concept continues to evolve.
“There has been significant progress in our understanding of the importance of nicotinamide mononucleotide (NMN), a key NAD+ intermediate, and nicotinamide phosphoribosyltransferase (NAMPT), particularly extracellular NAMPT (eNAMPT).”
The latest update, NAD World 3.0, introduces new insights into the roles of NMN, eNAMPT, and inter-tissue communication in aging.
“With these exciting developments, the further reformulated version of the concept, the NAD World 3.0, is now proposed, featuring multi-layered feedback loops mediated by NMN and eNAMPT for mammalian aging and longevity control.”
Key Points
Recent discoveries about NMN and NAMPT have expanded the NAD World concept, leading to several important updates:
- Extensive research has solidified Slc12a8’s role as a dedicated NMN transporter
- Small intestine has been recognized as a “signal generator” in the NAD World
- Slc12a8 levels adjust based on the body’s NAD+ needs
- NMN transporters in neurons facilitate brain-muscle communication
- eNAMPT-EVs deliver eNAMPT to specific tissues, boosting NAD+ production
- The efficiency of the eNAMPT-EV delivery system decreases as we age
- The NAD+ synthesis systems have distinct time and tissue preferences
- The hypothalamus plays a central role in NAD+-mediated aging regulation
The NMN Transporter (Slc12a8) is A Key Player in NAD+ Metabolism
Substantial evidence has confirmed Slc12a8’s role as a specific NMN transporter:
- Blocking Slc12a8: When Slc12a8 was blocked in cells or the small intestine, NMN uptake and NAD+ levels reduced significantly. Liver cells lacking Slc12a8 took up 90% less NMN.
- Boosting Slc12a8: Increasing Slc12a8 in cells or the small intestine raises NAD+ production, while lowering Slc12a8 leads to reduced NAD+ levels.
- Slc12a8’s Preferences: Slc12a8 is specific to NMN. It doesn’t transport NR or other similar compounds.
- Slc12a8 and Aging: Slc12a8 expression increases in the aged small intestine when NAD+ levels drop. If this increase is blocked, older mice can’t maintain normal NAD+ levels in their gut.
“Extensive biochemical and physiological characterizations of Slc12a8 led us to the conclusion that Slc12a8 is an NMN transporter.”
Previously, it was thought that NMN in the bloodstream was produced by the enzyme eNAMPT, but research shows eNAMPT is encapsulated in EVs and does not release NMN.
“Therefore, it is very likely that NMN in blood circulation is not the product of the extracellular enzymatic reaction.”
Thus, circulating NMN likely originates from intestinal absorption via the Slc12a8 transporter, not extracellular synthesis.
“We now hypothesize that NMN in blood circulation is maintained by the uptake of NMN through Slc12a8 in the small intestine.”
The Small Intestine, a “Signal Generator,” is the Fourth Key Tissue in the NAD World
Previously, three key tissues had been identified in the NAD World: the hypothalamus (the brain’s aging control center), skeletal muscle (a mediator), and adipose tissue (a modulator).
Following the discovery of Slc12a8, the small intestine has been recognized as a fourth crucial tissue, acting as a “signal generator” in the NAD+ World.
- Essential for NMN Uptake: In mice, lowering Slc12a8 levels in the gut drastically reduces NMN absorption, suggesting that the small intestine is the main site for NMN uptake.
- Influences Systemic NAD+ Levels: Research suggests gut bacteria may produce NMN, which is then absorbed through the intestine and contributes to NAD+ levels throughout the body.
“It is very likely that NMN is taken up through Slc12a8 in the small intestine and brought into blood circulation in the order of minutes, and distributed to many tissues and organs, including the pancreas, throughout the body.”
“We speculate that the small intestine can control the uptake of NMN as a systemic signaling molecule, providing another layer of regulation for NAD+ homeostasis throughout the body.”
NAD+ Precursor Absorption Adapts to Cellular and Whole-Body Needs
Another significant finding from the research is that NAD+ levels are carefully regulated through feedback mechanisms locally (within cells) and systemically (across the whole body).
- Slc12a8 Feedback: Slc12a8 is increased when NAD+ levels are low to enhance NMN absorption and reduced when NAD+ levels are sufficient to prevent excess uptake.
- NR Feedback: A similar feedback mechanism might exist for NR uptake, as cells lacking the NMN transporter tend to take up more NR to compensate.
“These observations strongly implicate that multiple feedback mechanisms exist to maintain tissue NAD+ homeostasis, and such feedback mechanisms seem to become more important in aged animals to counteract age-associated NAD+ decline.”
NMN Transporter Mediates The Brain-Muscle Connection
Neuronal NMN transporters (Slc12a8) are key for brain-muscle communication and play a crucial role in age-related muscle loss.
In mice, reducing the NMN transporter in the brain leads to metabolic and muscle decline, while restoring it improves energy metabolism, muscle mass, and strength.
“These findings strongly suggest that the inter-tissue communication between the LH and skeletal muscle plays a critical role in the maintenance of skeletal muscle mass and function during the process of aging, and Slc12a8-positive LH neurons most likely mediate this particular inter-tissue communication.”
eNAMPT-EVs: A Vital Delivery System for NAD+ in Target Tissues
Another key player in the NAD World is eNAMPT-containing extracellular vesicles (eNAMPT-EVs).
These tiny vesicles act as delivery vehicles, transporting eNAMPT, the enzyme that produces NMN, to target cells, enhancing NMN and NAD+ production.
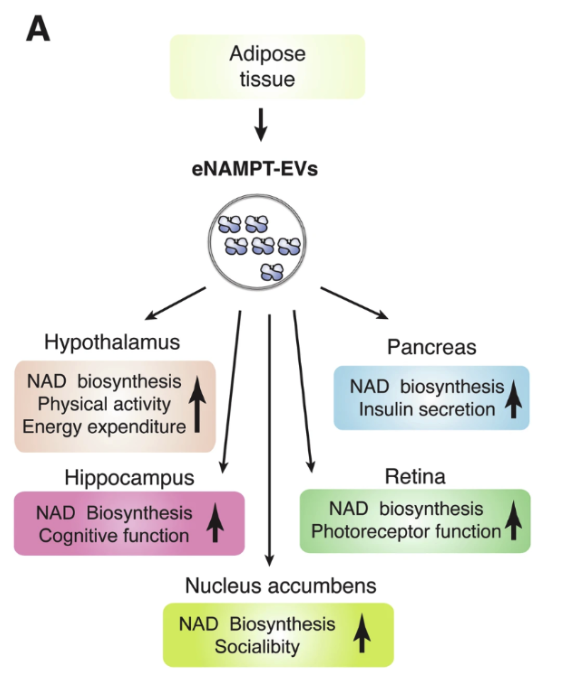
Fat tissue is a significant source of circulating eNAMPT-EVs, accounting for about half in circulation.
Illustrated in the figure, the release of eNAMPT from fat is tightly controlled and is important for maintaining NAD+ balance in target tissues, including the hypothalamus, hippocampus, nucleus accumbens, pancreas and retina.
“Although the detailed mechanism of eNAMPT encapsulation into EVs still remains unclear, this EV-mediated eNAMPT delivery system plays a critical role in maintaining NAD+ homeostasis in multiple tissues and organs under various pathophysiological conditions.”
eNAMPT-EV Delivery System Declines With Age
The efficiency of the eNAMPT-EV delivery system declines with age and is associated with reduced NAD+ levels and age-related functional decline.
“The age-associated decline in circulating eNAMPT levels causally contributes to the reduction in tissue NAD+ levels and SIRT1 activity, leading to the physiological decline in various tissue functions and producing aging phenotypes.”
Importantly, this functional decline can be improved through increasing circulating eNAMPT.
“eNAMPT-EV-administered aged mice look much healthier with shinier and richer fur and much more active, compared to vehicle-administered age-matched control mice.”
“Taken together, these findings clearly demonstrate the importance of the EV-mediated eNAMPT delivery system in the regulation of aging and longevity in mammals and the potential of eNAMPT-EVs as an effective agent to counteract age-associated functional decline.”
Timing and Tissue Preference Differ Between the NAD+ Synthesis Systems
The NAD World 3.0 concept proposes two main pathways for NAD+ production, each with its own characteristics and potential purpose:
- NMN/Slc12a8: Absorption of NMN through the Slc12a8 transporter.
- A rapid response system, delivering NMN quickly (minutes to hours) for immediate NAD+ needs.
- eNAMPT-EVs: EVs deliver eNAMPT to specific tissues.
- A long-term regulatory system, delivering eNAMPT more slowly (hours to days) for sustained NAD+ production.
Different tissues respond differently to NMN and eNAMPT. The liver, for example, responds strongly to NMN, while the hypothalamus prefers eNAMPT-EVs.
“Thus, differential utilization of these two NAD+ biosynthesis systems might comprise distinct, time-sensitive regulations of NAD+ biosynthesis in key tissues and organs that are involved in mammalian aging and longevity control.”
The Brain as the Control Center of Aging
The NMN/Slc12a8 and eNAMPT-EV pathways for NAD+ production each converge on the hypothalamus, highlighting its central role in NAD+-mediated aging regulation.
“Lastly, it should be emphasized that both NAD+ biosynthesis systems target the hypothalamus, the control center of aging in mammals, suggesting that maintaining proper NAD+ levels in the hypothalamus is essential to maintain biological robustness and counteract aging.”
Different neuron populations in the hypothalamus form feedback loops with key tissues:
- Fat Tissue & eNAMPT: Hypothalamic neurons regulate fat tissue function and stimulate eNAMPT secretion, which helps maintain energy balance, increase physical activity, and extend lifespan.
- Muscle & NMN: Slc12a8-positive neurons in the lateral hypothalamus (LH) influence skeletal muscle function through NMN signaling, impacting muscle health and aging.
Interestingly, high levels of NMN have been found in human breast milk, correlating with infant development, suggesting an early-life role for the NMN/Slc12a8 pathway.
“These findings raise an interesting possibility that infants uptake NMN from their mothers’ breast milk and utilize the NMN/Slc12a8-mediated NAD+ biosynthesis system for their development.”
The figure below illustrates the communication between tissues via NMN/Slc12a8 and eNAMPT-EVs to control aging and longevity.
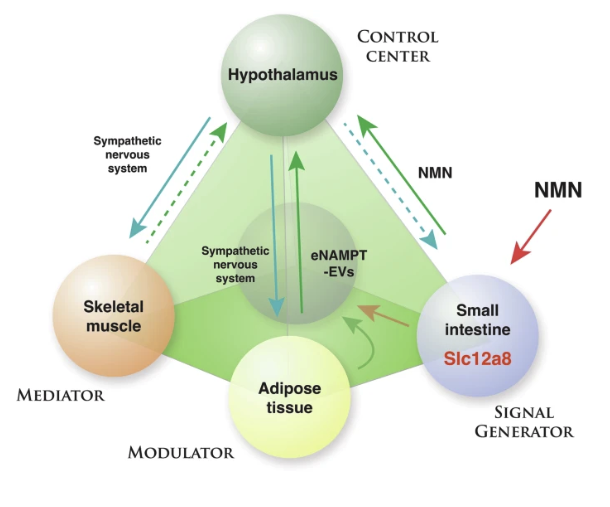
Four key tissues in the regulation of mammalian aging and longevity: the hypothalamus (control center of aging), skeletal muscle (a mediator), adipose tissue (a modulator), and the small intestine (a signal generator). Feedback loops between the hypothalamus and the three peripheral tissues modulate the system. NMN/Slc12a8 and eNAMPT-EVs are key regulatory components in these feedback loops.
Conclusion
The NAD World concept has advanced significantly, now in its 3.0 form, emphasizing the critical roles of the NMN transporter (Slc12a8) and eNAMPT-containing extracellular vesicles (eNAMPT-EVs) in age-related NAD+ maintenance.
The discovery of Slc12a8 as a dedicated NMN transporter highlights its role in maintaining NAD+ levels, with the small intestine emerging as a key site for NMN absorption and systemic NAD+ regulation.
Meanwhile, eNAMPT-EVs provide an alternative pathway, delivering eNAMPT to specific tissues and boosting NAD+ production.
The hypothalamus plays a central role in integrating NAD+ signals from these systems, influencing key processes related to aging, metabolism, and longevity.
By mediating inter-tissue communication, these pathways ensure that NAD+ production aligns with cellular and systemic demands.
These discoveries offer potential therapeutic targets for age-related NAD+ decline and healthy aging.
“Once we fully elucidate how each key inter-tissue communication works, we should be able to start developing effective anti-aging interventions that target and control the main regulatory factors in each feedback loop, such as NMN and eNAMPT-EVs.”
