Key Points
- Microscopy confirmed liposome formation and uptake by cells
- Liposomal NMN and HNK enhanced anticancer activity compared to free form
- The liposomes were safe, stable, and effectively released NMN and HNK
- A new HPLC method was established to quantify NMN encapsulation
NMN and HNK were Effectively Loaded into Liposomes
The effect of NMN encapsulation on liposome particle size and distribution was measured by dynamic light scattering (DLS). and is shown in the figure below.
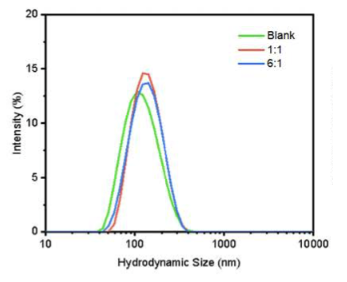
Different ingredient ratios were tested to find the best way to put NMN into the liposomes.
This figure shows particles without NMN (green line) had the smallest size. When NMN was added as the 1:1 preparation (red line) and the 6:1 preparation (blue line), the particle size increased, indicating encapsulation.
The 1:1 preparation proved most effective for incorporating NMN into liposomes, and it was selected for co-encapsulation with HNK.
A new HPLC method was used to determine the encapsulation efficiency of NMN and HNK.
“This method showed a clear peak shape of NMN with good reproducibility, and the method was characterized by good separation, simplicity, rapidity, specificity, and sensitivity.”
Electron Micrographs Confirms Liposome Formation
Electron Micrographs of NMN liposomes (left) and co-encapsulated (NMN and HNK) liposomes are pictured below.
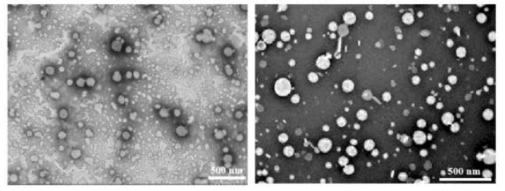
The liposomes were spherical with sizes ranging from 50-150 nm, consistent with measurements obtained from dynamic light scattering (DLS).
Liposomes Were Taken Up By Cells
A specialized technique was used to show the uptake of the NMN and HNK co-encapsulations in human colon cancer cells (HCT-116 cells) after 4 hours of incubation.
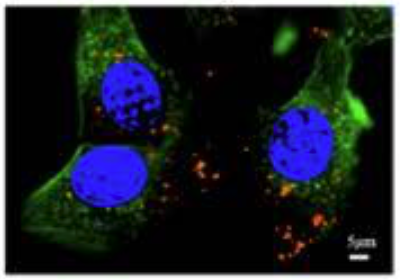
This image shows HCT-116 tumor cells’ nucleus (blue) and structure (green). The liposomes (red) are seen within the cells.
“Which indicated that the NMN@HNK@LNPs were efficiently uptaken intracellularly.”
Anticancer Effect Enhanced By Liposomal Encapsulation
The researchers studied the effects of NMN and the cancer drug HNK on human colon cancer cells (HCT-116 cells) in two ways: free compounds and co-encapsulated in liposomes. The figure below shows that, at an equivalent concentration, the ability to inhibit cancer cells is much more robust in liposomes (blue bars) compared to the free compounds (purple bars).
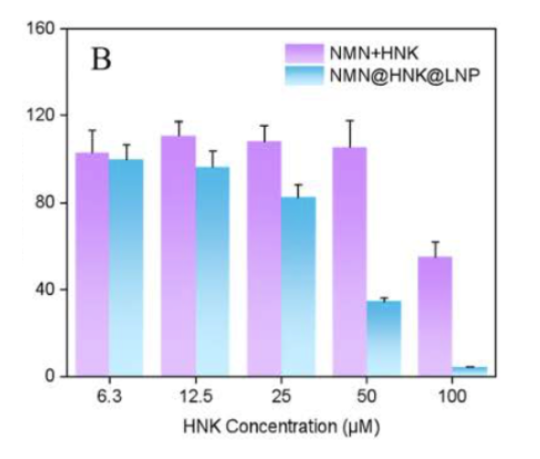
“NMN@HNK@LNP was significantly higher than HNK free drug in terms of cancer inhibitory activity.”
The Liposomes Were Stable, Efficiently Released, and Safe
The safety of the NMN and HNK liposomes was tested with red blood cells. Even at higher concentrations, the liposomes were deemed safe.
“The findings illustrated the high biosafety of NMN@HNK@LNPs.”
The release of NMN and HNK from liposomes was tested in conditions mimicking tumor cells and healthy cells: HNK was released faster in the healthy cells, while NMN was released at a more rapid rate in both environments.
Differences in their release from liposomes is likely due to the chemical properties of the molecule: NMN prefers water, whereas HNK prefers fat.
Conclusion
A novel microfluidic membrane emulsification device was used to co-encapsulate NMN and the anticancer drug, HNK.
Encapsulation efficiency was confirmed using a new HPLC method of analysis.
“A new high-performance liquid chromatographic method was built up to determine the encapsulation rate of NMN liposomes, which would be useful for the development of NMN drug applications.”
Liposomal delivery enhanced the anticancer effect in human colon cancer cells.
“Its inhibitory activity against colon cancer cells was significantly higher than that of the free drug.”
