This recent study found that in endothelial cells, the extracellular conversion of NMN to NR by CD73 enzyme in the cell membrane is a key mechanism to maintain intracellular NAD+
Like prior studies (1), they found that this transport method did not impede NMN from entering cells, as NR and NMN performed equally at restoring NAD+.
They also determined that when the NAD+ levels are low and cannot be maintained by internal recycling of the salvage pathway, endothelial cells increase production of CD73 to capture more NMN from the bloodstream.
* Endothelial cells form a single cell layer that lines all blood vessels and regulates exchanges between the bloodstream and the surrounding tissues.
Findings covered below are:
- CD73 is required to transport NMN in endothelial cells
- NMN is not impeded on entering cells
- NMN crosses cell membrane as readily as NR
- CD73 increased when NAD+ levels low
- Increased CD73 indicates importance of NMN for restoring vascular function
- NMN is available in blood and can be increased with supplementation
- NR is not available in blood
- NR Supplements do not increase NR level in the bloodstream
- Increased CD73 is necessary because NR is not available in bloodstream
Some researchers who favor Nicotinamide Riboside (NR) for restoring NAD+ claim that the need for CD73 to transport NMN inside cells means NR is more effective, as it can enter cells without CD73. Our takeaway from this research is actually very different. If NR was available, there would be no need to increase CD73.
The increased CD73 found in this study is because NR is not available, so is not sufficient to restore NAD+. NMN is available, can be increased with supplementation, is readily used by cells, and actively sought by cells to restore NAD+.
CD73 is required to transport NMN in endothelial cells
This is not new, as several studies have suggested or proven this in various other types of cells. We do not know if this is true in all cell types, as the authors state here:
“in cardiomyocytes, it was demonstrated that connexin 43 (Cx43) channels are permeable to extracellular NAD suggesting that intracellular transport of NAD and NMN may be cell-type dependent and reliant on various transporters”
Confirms that NMN is not impeded on entering cells
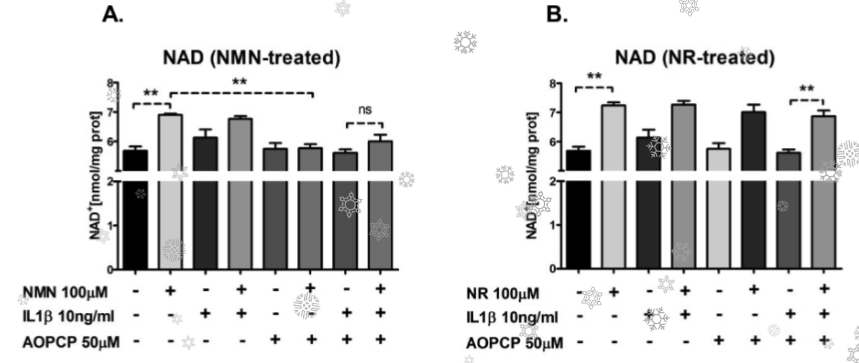
This research found that NMN is not impeded in entering cells to restore NAD+. You can see from the chart above that NMN restores NAD+ as well as NR, when placed outside cells.
According to the authors:
“Effects of NMN, that was comparable to the effects of NR in endothelial cells”
“Beneficial effects of NMN and NR were comparable …. as suggested in previous studies”
Remember, this was in cells where they placed NMN or NR outside the cells to see how they enter cells to restore NAD+. They performed equally – NMN was not disadvantaged.
In the body, NR is not found in the bloodstream at more than trace levels (below), so it is not that relevant anyway, except to dispel the argument that NR is a better precursor.
Other research showing NMN crosses cell membrane as readily as NR
This current study confirms other research showing the same results. For instance, the 2019 research below examines what happens to NR, NMN and other metabolites of NAD+ in the bloodstream and how they affect intracellular NAD+ levels in peripheral tissues.

When added to the serum outside of cells, all NAD+ metabolites were effective at restoring the NAD+ levels and metabolic activity INSIDE the cells. NMN was the most effective.
Interestingly, the conversion does not impact the ability to enter the cells.
They found CD73 enzymes on the outside of the cells themselves (ecto-enzymes) perform this conversion and are ubiquitous.
For example, the ecto-enzyme CD73 can cleave NAD+ to NMN and AMP, and also dephosphorylate the NMN to NR and adenosine.
Researchers introduced various NAD+ metabolites to the serum outside of cells.
As shown in the chart above, adding NAD+ or it’s metabolites to the serum OUTSIDE the cells were all effective at restoring the NAD+ levels and metabolic activity INSIDE the cells.
In fact, NMN was by far the most effective at restoring metabolic activity INSIDE the cells.
According to the authors:
Interestingly, exogenous nucleotides including NMN, NAMN, NAD+ and NAAD can support the maintenance of intracellular NAD pools as well as the nucleoside NR.
Moreover, the human ecto-enzyme CD73 has been described to catalyze both the cleavage of NAD+ to NMN and AMP as well as the subsequent dephosphorylation of the mononucleotides to the corresponding nucleosides, NR and adenosine.
NMN is not impeded, and NR is not better able to restore NAD+ inside cells.
CD73 increased when NAD+ levels low
In this research, cells were treated with H2O2, which increases inflammation and ROS, depleting NAD+ levels. They found cells respond by increasing NAMPT to power more recycling of NAD+ internally, and, increasing CD73 enzyme which reaches outside the cells to bring in NMN that is converted to NAD+.
According to the authors:
“CD73 and NAMPT expression in HAEC stimulated by IL1β were increased”
“This data suggests the compensatory upregulation of NAMPT, CD73 in response to inflammatory stimulus,might contribute to the preservation of NAD pool”
Increased CD73 indicates importance of NMN for restoring vascular function
Cells increase CD73 to grab more NMN from the bloodstream because NMN IS normally present in blood.
If NR was abundant in the bloodstream, endothelial cells would simply get the NR directly, without having to produce CD73 to transport NMN.
CD73 is increased to transport NMN BECAUSE NR is not available.
According to the authors:
“the local extracellular concentration of NMN may reach micromolar concentration range as suggested by some authors. Therefore, CD73, a well-known enzyme responsible for the conversion of AMP into adenosine and inorganic phosphate may represent an important regulatory pathway for extracellular NMN metabolism in endothelial cells.”
Other studies show NR is not bioavailable in blood
Below are 2 studies that show NR is not stable and not available in bloodstream of humans or mice.
Study #1:
This chart is derived from the Brenner study published in August 2019. NR is found only at trace levels in the bloodstream, and is not increased after supplementation of 1,000 mg of NR per day for 3 weeks.
- NR was found at trace levels in the blood
- NR levels were unchanged after supplementation
- MeNAM and Me2PY are the primary result of NR supplementation, not NR

Study #2:
The chart at left is from the Bauer study and shows the change in NR, NAM and NMN found in blood plasma after oral gavage of NR at 200 mg/kG (7).
NR was found at trace levels and not increased.
Within minutes, NAM was increased 16x, reaching 40x increase by 100 minutes.
This is a short-term increase in NAM following a single dose of NR, which shows it is degraded to NAM. This increased NAM is temporary, as excess NAM will be methylated and excreted in urine (see below).
According to the authors:
Oral NR dosing increased circulating NAM 40-fold while NMN remained unchanged and NR was detected only at trace levels in the blood.
Orally administered NR that reaches the muscle appears to enter in the form of liberated NAM.
Increased CD73 not necessary if NR was available in bloodstream
This study shows Endothelial cells upregulate production of CD73 when desperate for NAD+.
If NR was available in the bloodstream, there is no need to send out CD73 to grab NMN from the bloodstream.
CD73 transport of NMN vs direct uptake of NR
Some researchers who favor Nicotinamide Riboside (NR) for restoring NAD+ point to this and similar research, and claim the need for CD73 to transport NMN inside cells means NR is more effective, as it can enter cells without CD73.
But the details of this study tell a different story.
- NMN is not impeded at entering cells to restore internal NAD+
- CD73 is increased in response to low NAD+ levels to bring more NMN inside the cell
- NR can not be effective at restoring NAD+ in endothelial cells as it is never available in blood
- CD73 would not be needed to bring NMN inside cells if NR was available
Conclusion
CD73 is upregulated to bring more NMN into cells that are low on NAD+ precisely because NR does not survive in the bloodstreamand is unavailable.