This study examined the effects of pretreating mice with EGCG to delay destruction of dopamine-sensitive brain cells to promote mobility and stable moods.
EGCG showed several benefits in mice with damaged neurons:
-
- Reduced anxiety-like behaviors and motor impairment
- Reduced degeneration of key brain cells controlling movement
- Lowered accumulation of harmful proteins linked to destruction of brain cells
- Protected neurons through modulating inflammatory response
Injected EGCG to Combat Loss of Muscle Control in Mice
A mouse model with induced damage to dopamine-sensitive brain cells was used to explore the effectiveness of EGCG in protecting muscle movement control and mood stability.
EGCG was delivered by injection at a dose of 10 mg/kg.
“In this study, the neuroprotective properties of EGCG on the chronic progression of PD were assessed by constructing, for the first time, a chronic mouse model generated by PFFs subjected to EGCG pretreatment.”
Mice were divided into three groups:
-
- Saline (control): Healthy mice, did not receive EGCG
- Model group: Mice with damaged neurons, did not receive EGCG
- Treatment group: Mice with damaged neurons, received injection of EGCG prior to neuron damage
EGCG Protects Muscle Function and Mood
EGCG pretreatment improved muscle control and mood stability after induced brain cell damage.
“Long-term and multiple behavioral experiments revealed that EGCG ameliorates the anxiety of [Parkinson’s Disease] mice, enhances the spontaneous motor ability of mice, and improves muscle strength and the function of motor coordination.”
Mice receiving EGCG exhibited significantly reduced symptoms of anxiety and maintained muscle control and strength throughout the 6-month experiment.
EGCG Preserves Dopamine-Sensitive Neurons
Mice in the EGCG group exhibited significantly slower and less pronounced damage to dopamine-sensitive neurons responsible for motor control and mood regulation.
“Parkinson’s disease (PD) is the second most common neurodegenerative disease characterized by the progressive degeneration of dopaminergic neurons in the substantia nigra (SN).”
The figure below shows the relative protection of dopamine-sensitive neurons in the three groups.
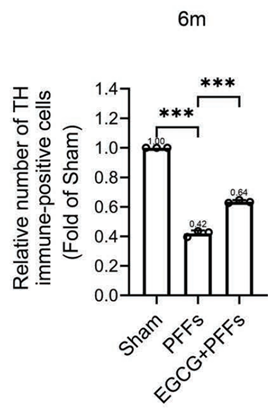
In this figure, the white bars represent the number of TH immune-positive cells, a measure of healthy dopamine-sensitive cells.
Mice with damaged brain cells (PFFs group) experienced a 58% decrease in dopamine-sensitive cells compared to healthy mice (Sham group), where the EGCG pretreatment group (EGCG+PFFs group) only experienced a 36% loss in these cells.
EGCG Injection Slows Damaging Protein Accumulation
EGCG pretreatment prevented the accumulation of α-synuclein preformed fibrils, a type of protein associated with Parkinson’s Disease and known to severely impair brain function.
The figure below shows the accumulation these proteins in the brain tissue of the mice.
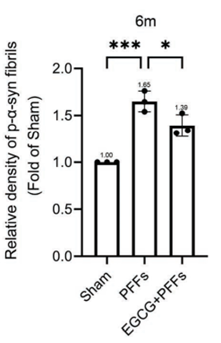
The mice with damaged neurons (PFFs group) experienced a 65% increase in the harmful protein compared to healthy mice (Sham group).
The mice in the EGCG pretreatment group (EGCG+PFFs group) experienced only 39% increase, showing the protective effect of EGCG.
EGCG Combats Nerve Damage and Inflammation
Levels of proinflammatory molecules were significantly lower in the ECGC pretreatment group, suggesting a mechanism by which EGCG protects against inflammation in the brain.
“The results of our study indicated that EGCG exerted anti-inflammatory properties by inhibiting the release of pro-inflammatory cytokines and promoting the release of anti-inflammatory cytokines at the transcriptional level.”
Conclusion
Using EGCG as a pretreatment prior to dopamine-sensitive nerve damage protected neurons, reduced inflammation, improved behavioral tests in mice.
The study suggests that EGCG was an effective way to protect brain tissue and preserve motor function and mood stability.
“These results suggest that EGCG pretreatment may alleviate PFF-induced neuroinflammation in the nigrostriatal pathway, thus exerting a neuroprotective role in a [Parkinson’s Disease] mouse model.”
