Intercellular communication: communication between cells
All species of animals, including the human being, are multicellular organisms (an organism that consists of more than one cell). In order to work collectively and maintain the homeostasis across multiple tissues, our cells have developed multiple different ways to receive, process, and transmit information between each other, including endocrine, neuroendocrine, and neuronal signaling. It is a fundamental property of all cells in every living multicellular organism which contributes to the homeostasis of all tissues and many normal physiological functions.
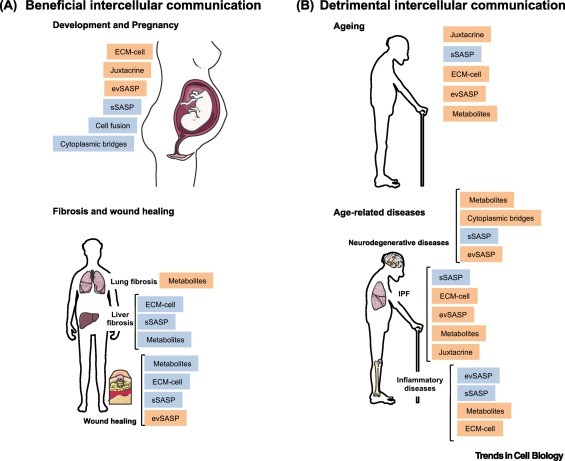
Altered Intercellular communication during aging
Like many other important functions, intercellular communication behavior is altered during the aging process. One of them is neurohormonal signaling. The neurohormones are hormones that are produced and released by neuroendocrine cells (also called neurosecretory cells) into the blood. Therefore, the neurohormones play critical roles in the neuronal regulation of metabolism. Previous studies have shown that the neurohormonal signaling (e.g., renin-angiotensin, adrenergic, insulin-IGF1 signaling) tends to be deregulated in aging. In contrast, inflammatory reactions increase, immunosurveillance against pathogens and premalignant cells decline, and the composition of the peri- and extracellular environment changes (Lopez-Otin et al., 2016).
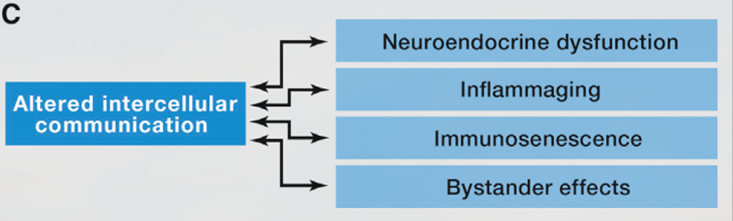
Inflammaging
The most prominent age-associated change in intercellular communication is increased inflammation. It is so important in many of age-associated diseases that researchers have specifically termed it “inflammaging”. Inflammaging can be caused by various factors. For instance, the accumulation of proinflammatory tissue damage, or a declined immune system that failed to effectively remove the pathogens (like bacteria) or dysfunctional host cells (especially cancerous cells and senescent cells).
Another big player in inflammaging are senescent cells (see previous Hallmarks of Aging article on cellular senescence). Senescent cells actively secrete various proinflammatory cytokines, which are known as the “SASP factors” (senescence-associated secretory phenotype). These factors can induce local inflammation. These alterations result in the activation of inflammasome and other proinflammatory pathways and eventually, lead to increased production of inflammatory molecules like IL-1b, tumor necrosis, and interferons.
Inflammation is also a major contributor to obesity and metabolic diseases like type II diabetes, which significantly contribute to and correlate with aging in the human population (Barzilai et al., 2012). Similarly, the increased inflammation is also a major contributor to atherosclerosis.
Immunosenescence
Together with inflammaging, the function of adaptive immune response also declines during aging. This phenomenon is known as immunosenescence (the aging of the immune system). Immunosenescence is caused by the accumulation of damage to the immune system during aging, thus resulting in the inability of the immune system to effectively remove pathogens and infected cells. This is also one of the major reasons that the COVID19 pandemic has caused a significant number of deaths in the elder population.
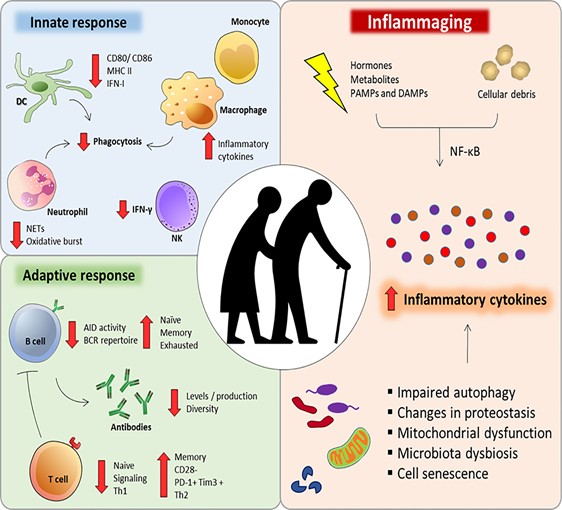
Chronic inflammation causes a reduction in NAD+
Chronic inflammation induces the proliferation of macrophages, a type of white blood cell of the immune system that engulfs and digests the pathogenic particles, including cancer cells, microbes, and foreign substances. More importantly, the macrophages have high expression of CD38 protein, which degrade NAD+ and thus, NAD+ levels are reduced.
NAD+ precursor attenuates inflammation
In April 2021, a Kang et al. investigated the effects of nicotinamide riboside (NR) on inflammation and oxidative stress in macrophages (Kang et al., 2021). They found that NR significantly attenuates the inflammatory gene expression in macrophages. In addition, NR also abolished the accumulation of cellular reactive oxygen species.
Although this study only provides limited evidence, it is a good indication that NAD+ precursors might be a good candidate for the treatment of inflammaging and immunosenescence. Future clinical trials need to be conducted to determine the efficacy of NAD+ precursors on age-related immune system disorders.
